Abstract
Introduction: CD28 or 4-1BB co-stimulated CD19 chimeric antigen receptor (CAR) T-cell has demonstrated remarkable outcomes in B-cell malignancies. Recently, we reported that a novel CD19CAR-T cell incorporated B-cell co-stimulatory molecules; CD79A/CD40 (CD19.79a.40z), exhibited superior anti-tumor activity in B-cell lymphoma model compared to CD28 or 4-1BB co-stimulated CAR-T cell. Despite these promising results, the molecular signatures of this novel CAR-T cell and its effects on the overall cellular state are not well understood. This study assessed mRNA expression using RNA-sequencing (RNA-seq) to interrogate the intrinsic transcriptional gene underlying CD19.79a.40z CAR-T cell response and identify the differentially expressed genes (DEGs) compared to CD19.28z or CD19.BBz CAR-T cell upon CD19-specific antigen exposure.
Methods: We generated three CD19CAR structures contained different co-stimulatory domain (CD19.28z, CD19.BBz, CD19.79a.40z).The CAR genes were transfected into retroviral packaging cells and transduced into human CD3+ cells. We first screened the functions of each CAR-T cell structure in in vitro assay including cytotoxicity assay, T-cell proliferation assay, and T-cell phenotype assay to confirm whether each CAR-T cell structure contained proper functions. Total CAR-T 3 - 6 x 106 cells were harvested and extracted for RNA at post-stimulation with the target cell line on day seven. Nine samples were RNA-seq using Illumina Hiseq 4000. The short sequence reads were mapped to reference genome from GENCODE (GRCh38) and the expression count matrix was then computed using HTSeq. EdgeR program was used to perform DEGs analysis between three groups: CD19.79a.40z vs. CD19.28z, CD19.79a.40z vs. CD19.BBz and CD19.28z vs. CD19.BBz. The DEGs were selected at FDR <0.05 and fold change ≥ 1.5-fold difference. To identify the regulation of functionally enriched pathways, we performed both ranked gene list analyses on the whole gene expression level using the gene set enrichment analysis (GSEA) software from the Broad Institute and DEGs list separately analyzed for up- and down-regulated genes using g:Profiler.
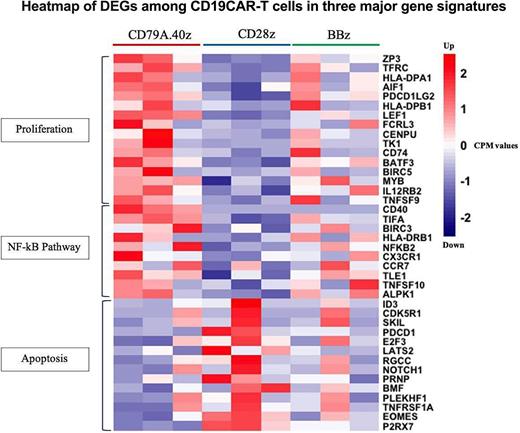
Results: A total of 374 DEGs (232 up-regulated and 142 down-regulated DEGs) and one up-regulated DEG were identified in CD19.79a.40z vs. CD19.28z CAR-T cell groups and CD19.79a.40z vs. CD19.BBz CAR-T cell groups respectively. We found 104 DEGs in which 32 DEGs were up-regulated and 72 DEGs were down-regulated in CD19.28z CAR-T cell compared to CD19.BBz CAR-T cell.Functional enrichment analysis using g;Profiler illustrated that CD19.79a.40z CAR-T cells consisted of down-regulated genes mediating apoptosis and exhaustion while up-regulated genes correlated with T-cell activation, proliferation, positive regulation of interferon production, and NF-κB pathway. Compared to either CD19.28z or CD19.BBz CAR-T cells, CD19.79a.40z CAR-T cells strongly enriched in genes associated with T-cell proliferation at the transcription level supported by the expression of BATF3, IL2, ZP3, TFRC,IRF1 and CD70. In addition, CD19.79a.40z CAR-T cell contained many down-regulated apoptosis-related genes, including ID3, PDCD1 and EOMES (Figure). GSEA revealed that CD19.79a.40z CAR-T cells enriched in both glycolysis and fatty acid metabolism, which are the underlying metabolic pathway closely linked to T-cell activation and proliferation. Regarding T-cell differentiation, CD19.79a.40z CAR-T cells were particularly enriched in naïve and memory-related genes compared to CD19.BBz CAR-T cell.
Conclusions: This study provides a comprehensive insight into the understanding of gene expression and its related pathways that potentiates the superior anti-tumor functions by CD19CAR-T cell incorporated B-cell co-stimulatory domain.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal